Nucleosynthesis
Nucleosynthesis is the creation (synthesis) of the elements of the periodic table (nuclides). All of the atoms—except hydrogen and helium—in your body, on Earth, and in Earth were created in stars. Some of the elements, like most carbon and oxygen, were created "hydrostatically" while the star was steadily burning nuclear fuel. Some of the elements, like iron, were created "explosively" in about one second during a supernova.
Our group studies nucleosynthesis through the lenses of galactic archaeology and galactic chemical evolution. We track the amount of an element over time in a galaxy, and we try to figure out where that element came from.
Iron, Nickel, and Manganese (Type Ia Supernovae)
For example, we know that most of the iron (Fe) in our solar system came from the explosions of white dwarfs (Type Ia supernovae). However, we don't know exactly how those white dwarfs explode. White dwarfs have a curious property that they must explode if they grow in mass to about 1.4 times the mass of the sun—the Chandrasekhar mass. If that is the only reason that white dwarfs explode, then they will they should produce copious amounts of manganese (Mn) and nickel (Ni).
Prof. Mia de los Reyes and I found dwarf galaxies have too little manganese and nickel for most white dwarfs to be exploding at the Chandrasekhar mass.
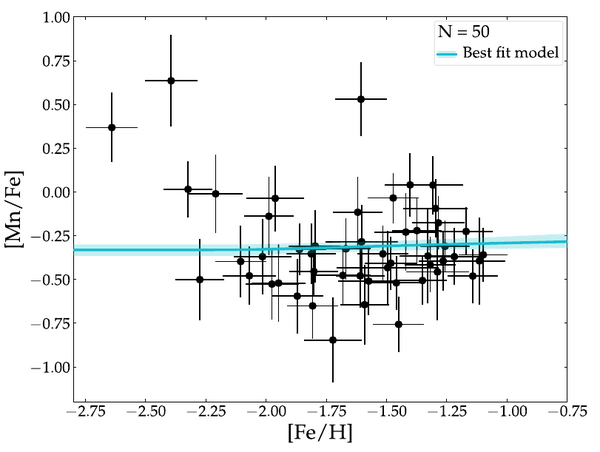
There is not much manganese in the Sculptor dwarf galaxy (de los Reyes et al. 2020). The solar system's ratio of manganese to iron is [Mn/Fe] = 0. Sculptor's manganese-to-iron ratio is about half of the solar value. We concluded that white dwarfs are exploding a low masses.
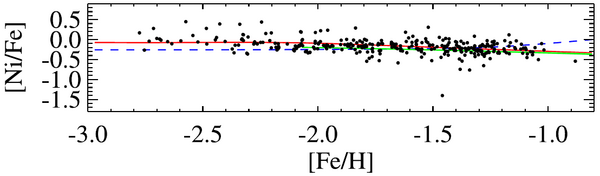
The nickel abundances in Sculptor also corrborate low white dwarf masses (Kirby et al. 2020). The solar nickel-to-iron ratio is [Ni/Fe] = 0. White dwarfs that exploded because they reached the Chandrasekhar mass would have [Ni/Fe] > 0.
The story is nuanced. Galaxies with more stars have larger manganese-to-iron and nickel-to-iron ratios. We believe that white dwarfs explode for a variety of reasons at a variety of masses. The bigger galaxies form stars over a more extended period of time, and we believe that Chandrasekhar-mass Type Ia supernovae require a long time (about one billion years) to explode. As a result, small galaxies like Sculptor did not form stars for a long enough period of time to witness any Chandrasekhar-mass white dwarf explosions.
Yttrium, Barium, Europium, and More (r-process)
The heaviest elements in the periodic table (atomic number greater than 30) are formed through the neutron-capture process. That process has a number of channels, most notably rapid (r-process) and slow (s-process). The heavy elements in globular clusters were made in the r-process. Within most globular clusters, the stars have the same amount of the r-process elements. The globular cluster M15 is unusual because the r-process abundances vary quite a lot from star to star.
We recently discovered that the globular cluster M92 also has r-process abundances that vary from star to star. Even more interestingly, this variation depends on the amount of magnesium (Mg) in the stars. The stars can be grouped into those with lots of magnesium (1G) and stars with little magnesium (2G). Stars in 1G have a large range of r-process abundances. Stars in 2G have constant r-process abundances.
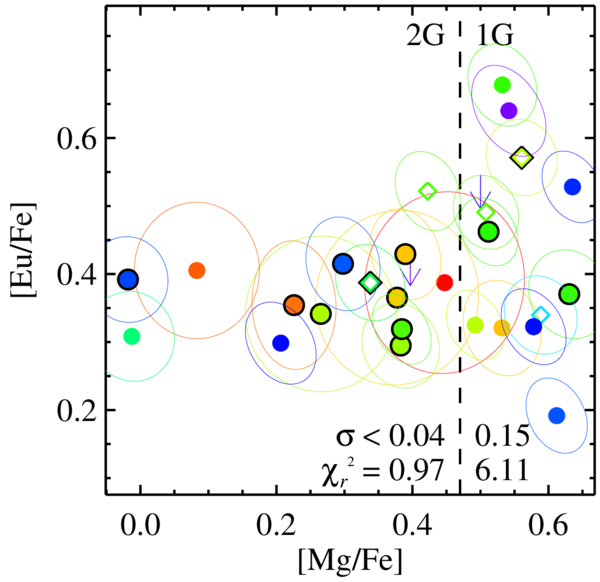
Our interpretation is that an r-process source—maybe a rare type of massive supernova—made a lot of r-process elements, like europium (Eu), concurrently with the formation of stars in 1G. As the stars and gas orbited the center of the cluster, the europium mixed evenly. By the time stars in 2G formed, the concentration of europium was the same everywhere in the cluster. This is like sprinkling salt into a pot of soup. If you don't stir the pot, then the soup will be too salty near the top of the pot and not salty enough at the bottom of the pot. After you stir a few times, the soup is evenly salted. Likewise, M92 needed to be "stirred" a few times (a few orbits around the cluster) before it became evenly "salted" with europium.